- Salt Water Pool and Spa
- Salt Water Pool Maintenance
- Langelier Saturation Index
Langelier Saturation Index
The langelier saturation index or LSI is a valuable tool to check the overall state of water and has been used in the pool industry for decades. It helps determine if water is generally corrosive, prone to scaling or in a good neutral state. It's extremely useful when troubleshooting or trying to prevent serious issues like scaling or rust formation in your pool that can lead to costly problems down the road.

Even though the LSI is a complex scientific formula we will break it down and simplify how it works. It was originally used by Dr. Wilfred Langelier in the early 20th century and has since been used by pool professionals around the world. As a pool owner or service professional it can be very helpful to quickly and accurately identify serious and costly issues with pool chemistry. If you've ever experienced scale buildup like that annoying white line along the surface or unsightly etching, this article will help you understand why it happens and how to prevent it.
Langelier Saturation Index Basics
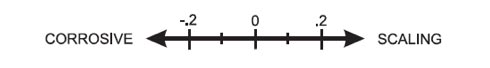
The Langelier Saturation Index is a simple way to determine if water has a negative LSI and is corrosive, or has a positive LSI and is scale-forming. It works on a sliding scale where -0.3 to +0.3 is an acceptable range for pool water with 0.0 being ideal. As water falls out of balance, it will also naturally try to reach neutral or 0.0, and this is where issues can arise. This includes releasing calcium that will cause scaling or corrosion that can damage etched plaster or corrosion of pool equipment and even plastics.
The release of calcium when the LSI is high is especially problematic for salt water pool owners as it will cause premature and increased calcium scale formation on the chlorinator. If you suspect your pool cell chlorinator has calcium formation it will need to be cleaned to ensure it's operating at peak chlorine production capacity.
Calculating LSI
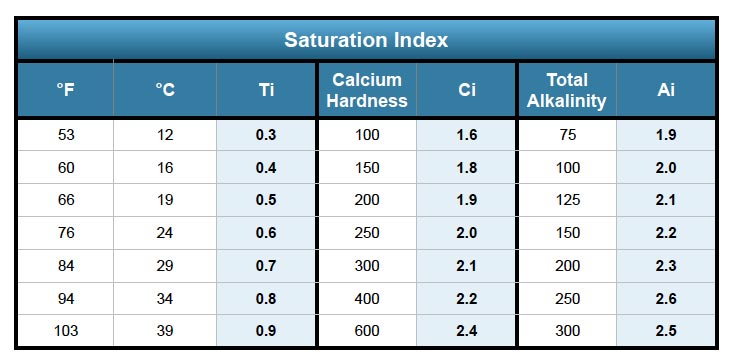
The first step in calculating the saturation index using the Langelier scale is determining 4 factors or variables and if you've ever tested pool water before or carried out your own pool maintenance this should be quite simple. You will need to obtain pH, Water Temperature (Ti), Calcium Hardness (Ci) and Total Alkalinity (Ai). We will take a sample pool so we can demonstrate how to input the numbers into the formula. The langelier saturation index equation will require some simple math but if math isn't for you, we recommend using an LSI calculator.
Saturation Index = pH + Ti + Ci + Ai - 12.1
LSI Calculation Sample #1 (Neutral)
pH: 7.4
Temperature (Ti): 84°F
Calcium Hardness (Ci): 200
Total Alkalinity (Ai): 100
Si = 7.4 + 0.7 + 1.9 + 2.0 - 12.1
Saturation Index -0.1
The sample pool above has a saturation index of -0.1 which falls into the acceptable range. We will know with a great degree of certainty that the water is not corrosive and should not form any scaling on pool surfaces or equipment. It is important to keep in mind that fluctuations in any of the above readings can push the water out of balance and cause things like algae blooms or cloudy pool water. In this sample we could add a slight amount of sodium bicarbonate to increase LSI but it's close enough to neutral to leave it and monitor.
LSI Calculation Sample #2 (Corrosive)
pH: 7.4
Temperature (Ti): 54°F
Calcium Hardness (Ci): 200
Total Alkalinity (Ai): 100
Si = 7.4 + 0.3 + 1.9 + 2.0 - 12.1
Saturation Index -0.4
In this sample we've demonstrated how fluctuating water temperatures can affect water chemistry. If the water temperature dropped 30°F in the sample pool above, a temperature of 54°F would result. This is very possible in cooler climates in the northern United States and Canada. The Langelier Saturation Index makes it easy to see that with the index in the negatives, we are at risk of corrosion to the pool hardware. In this sample we would want to add sodium bicarbonate to increase the LSI to the ideal range.
LSI Calculation Sample #3 (Scaling)
pH: 7.6
Temperature (Ti): 94˚F
Calcium Hardness (Ci): 250
Total Alkalinity (AI): 200
Si = 7.6 + 0.8 + 2.0 + 2.3 - 12.1
Saturation Index +0.6
In this sample calculation we can see that with a slightly higher water temperature, upper recommended levels of calcium hardness and alkalinity, the total LSI has risen substantially. In this pool we would typically start to see scaling on the pool walls, plumbing or the chlorinator. In this sample we would want to quickly add sodium bisulphate to lower the acidity and reduce the overall LSI.
Adjusting LSI Values
When pool water is balanced it should have virtually no negative effects on pool equipment or surfaces. If the value falls below -0.3 or above 0.3 you should take immediate action to prevent costly damage, unsightly stains or an unsanitary pool. We'll go over how to increase and decrease LSI but it's important to always double check your calculation before making adjustments or consult a pool professional.
Increasing LSI +
If the index value is less than -0.3 pool water is corrosive by nature. It could potentially lead to etching or dissolving of pool walls including grout and plaster. It could also cause stains that are time consuming to clean and could even become permanent if left for a long period of time.
Add sodium bicarbonate or baking soda to increase the Alkalinity and overall LSI value.
In The Swim Alkalinity Increaser Sodium BicarbonateAlkalinity increaser sodium bicarbonate for increasing LSI. If you click on this link and make a purchase, we may earn a commission. |
Decreasing LSI -
If the langelier saturation index is greater than 0.3 pool water is scale forming by nature. It will tend to deposit excess minerals on pool equipment, walls, plumbing and the salt cell itself. Scale will appear as white spots on pool walls, restrict water flow, reduce filtration quality and shorten the effectiveness and lifespan of the chlorine generator.
Add sodium bisulfate or muriatic acid to decrease the pH and overall LSI value.
In the Swim Sodium Bisulfate Acid pH and Alkalinity ReducerSodium bisulphate for reducing high pH and alkalinity levels. 1.5 pounds required per 10,000 gallons. If you click on this link and make a purchase, we may earn a commission. |
Disclaimer
Please use all appropriate and proper safety precautions when attempting projects on this website. All projects are attempted at the reader's own risk.
Salt Water Pool and Spa™ participates in the Amazon Services LLC Associates Program, as an Amazon Associate we may earn a commission from qualifying purchases.

